No2 Lewis Structure

Lone pairs are in green. Bond pairs are in red. NO2 lewis structure
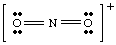
Figure %: Lewis Structure of NO2 +. To improve your skills in writing Lewis
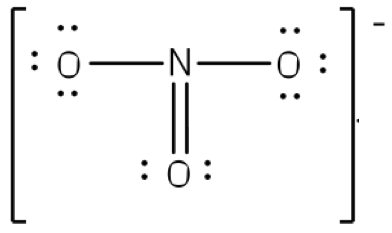
Lewis Structures for Resonance Structures

The symmetry group was detected using Serguei Patchkovskii's code
File:Nitrite-ion-lewis-canonical.png. Size of this preview: 800 × 189 pixels
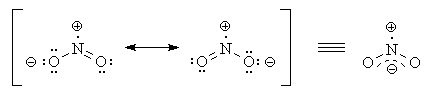
Re: What is the proper lewis structure for NO2, and its properties?
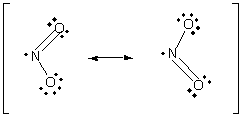
NO2 has an unpaired electron in its Lewis structure:

How many valence electrons in NO2- and NO2+?
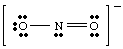
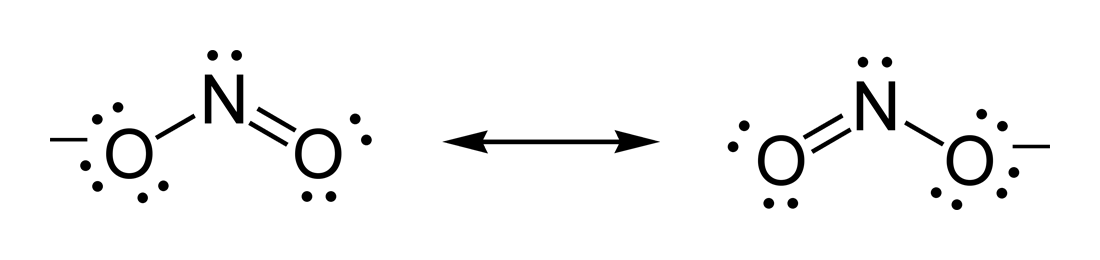
Figure %: NO2 -. If we had tried to draw the above structure without taking

Re: lewis structure of Urea
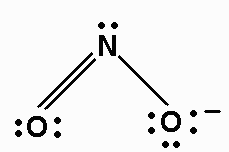
h) NO2- There are 18 valence electrons. The Lewis structure is:

NO2-Schema-Lewis.png (351 × 94 píxeles; tamaño de archivo: 3 KB;
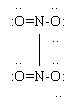
At the right is a Lewis structure for N2O4. Note that the octet rule is now
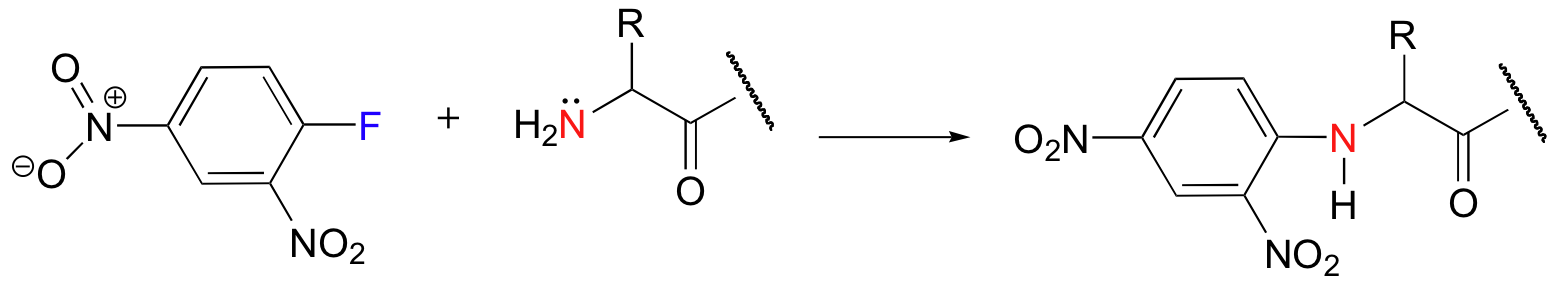
Lewis Structures - ChemWiki

that there are two equally valid Lewis structures that can be drawn: NO2

Re: lewis structure of Urea
Here is another example, using the molecule NO2:
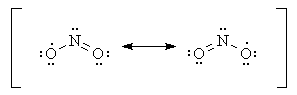
more resonance structures for NO2. The lone pair on nitrogen in these
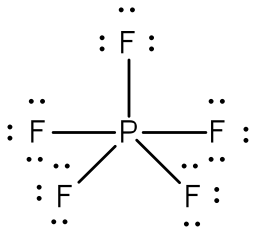
Lewis Structures for Electron-rich Compounds

Use the Lewis structure of the NO2 molecule shown in the figure below to